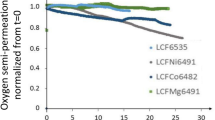
Abstract
Three dense membranes of types SrCo0.8Fe0.2O3−δ (SCF(82)), La0.6Sr0.4Co0.8Fe0.2O3−δ (LSCF(6482)) and La0.8Sr0.2Co0.6Fe0.4O3−δ (LSCF(8264)) perovskites were prepared by complexation applying a chelating agent, ethylene diamine N,N,N′,N′-tetra-N-acetyl-diamine (EDTNAD). The oxygen permeation flux through the perovskite membranes was measured as a function of temperature within 1,073–1,223 K as well as the oxygen partial pressure of 0.1–1.0 bar. The oxygen permeation fluxes for the membranes, SCF(82), LSCF(6482), LSCF(8264) with the thickness of 0.85 mm were observed as 9.2 × 10−7 (mol/cm2 s), 1.7 × 10−7 (mol/cm2 s), and 1.0 × 10−7 (mol/cm2 s) in these cases at 1,153 K. The results indicated the oxygen permeation process was mainly controlled by the oxygen bulk diffusion through these membranes.





Similar content being viewed by others
References
Zhu X, Sun Sh, He Y, Cong Y, Yang W (2008) New concept on air separation. J Membr Sci 323:221–224
ten Elshof JE, Bouwmeester HJM, Verweeij H (1995) Oxidative coupling of methane in a mixed-conducting perovskite membrane reactor. Appl Catal A 130:195–212
Liu M, Joshi AV, Shen Y, Krist K (1993) Mixed ionic-electronic conductors for oxygen separation and electrocatalysis. US Patent 5,273,628, 28 Dec 1993
Carlan MF, Dyer PN, Throgood RM (1993) Process for recovering oxygen from gaseous mixtures containing water or carbon dioxide which process employs ion transport membranes. US Patent 5,261,932, 16 Nov 1993
Wang HH, Cong Y, Yang WS (2002) Oxygen permeation study in a tubular Ba0.5Sr0.5Co0.8Fe0.2O3−δ oxygen permeable membrane. J Membr Sci 210:259–271
Balachandran U, Dusek JT, Maiya PS, Ma B, Mieville RL, Kleefish MS, Udovich CA (1997) Ceramic membrane reactor for converting methane to syngas. Catal Today 36:265–272
Wang HH, Cong Y, Yang WS (2003) Investigation on the partial oxidation of methane to syngas in a tubular Ba0.5Sr0.5Co0.8Fe0.2O3−δ membrane reactor. Catal Today 82:157–166
Tsai CY, Dixon AG, Moser WR, Ma YH (1997) Dense perovskite membrane reactors for the partial oxidant of methane to syngas. AIChE J 43:2741–2750
Wang HH, Cong Y, Yang WS (2002) Partial oxidation of methane to syngas in tubular oxygen-permeable reactor. Chin Sci Bull 47:534–537
Xu S, Thomson WJ (1997) Perovskite-type oxide membranes for the oxidative coupling of methane. AIChE J 43:2731–2739
Wang HH, Cong Y, Yang WS (2002) High selectivity of oxidative dehydrogenation of ethane to ethylene in an oxygen permeable membrane reactor. Chem Commun 14:1468–1469
Akin FT, Lin YS (2002) Selective oxidation of ethane to ethylene in a dense tubular membrane reactor. J Membr Sci 209:457–467
Wang HH, Cong Y, Yang WS (2002) Continuous oxygen ion transfer medium as a catalyst for high selective oxidative dehydrogenation of ethane. Catal Lett 84:101–117
Wang HH, Cong Y, Zhu XF, Yang WS (2003) Oxidative dehydrogenation of propane in a dense tubular membrane reactor. React Kinet Catal Lett 79:351–356
Minh NQ (1993) Ceramic fuel-cells. J Am Ceram Soc 76:563–568
Teraoka Y, Nobunaga T, Yamazoe N (1988) Effect of cation substitution on the oxygen semipermeability of perovskite oxides. Chem Lett 3:503–506
Mai A, Tietz F, Stfver D (2004) Partial reduction and re-oxidation of iron-and cobalt-containing perovskites using catalyst characterization measurement. Solid State Ion 173:35–40
Beckel D, Gyger UPM, Florey GA, Gauckler LJ (2007) Electrochemical performance of LSCF based thin film cathodes prepared by spray pyrolysis. Solid State Ion 178:407–415
Teraoka Y, Honbe Y, Ishii J, Furukawa H, Moriguchi I (2002) Catalytic effects in oxygen permeation through mixed-conductive LSCF perovskite membranes. Solid State Ion 152–153:681–687
Scott SP, Mantzavinos D, Hartley A, Sahibzada M, Metcalfe IS (2002) Reactivity of LSCF perovskites. Solid State Ion 152–153:777–781
Liu YF, Liu XQ, Meng GY (2001) A novel route of synthesizing La1−x Sr x CoO3 by microwave by microwave irradiation. Mater Lett 48:176–183
Li Sh, Jin W, Xu N, Shi J (2001) Mechanical strength, and oxygen and electronic transport properties of SrCo0.4Fe0.6O3−δ YSZ membranes. J Membr Sci 186:195–204
Jin W, Li Sh, Huang P, Xu N, Shi J (2001) Preparation of an asymmetric perovskite-type membrane and its oxygen permeability. J Membr Sci 185:237–243
Doorn RHEV, Burggraaf AJ (2000) Structural aspects of the ionic conductivity of La1−x Sr x CoO3-δ. Solid State Ion 128:65–78
Teraoka Y, Zhang HM, Furukawa S, Yamazoe N (1985) Oxygen permeation through perovskite-type oxides. Chem Lett 14:1743–1746
Burggraaf AJ, Cot L (1996) Fundamentals of inorganic membrane science and technology. Van Nostrand Reinhold, New York
Wagner C, Schottky WZ (1930) Beitrag zur Theorie des Anlaufvorganges. Phys Chem B11:25–41
Wagner C (1975) Equations for transport in solid oxides and sulfides of transition metals. Prog Solid State Chem 10(1):3–16
Zeng Y, Lin YS, Swartz SL (1998) Perovskite-type ceramic membrane: synthesis, oxygen permeation and membrane reactor performance for oxidative coupling of methane. J Membr Sci 150:87–98
Wang H, Yang W, Tablet C, Caro J (2005) Oxygen diffusion in oxide crystals tracing new routes to identify the rate limiting step of oxygen permeation through perovskite membranes. Diffus Fundam 2:4610–4615
Vente JF, Haije WG, Rak ZS (2006) Performance of functional perovskite membranes for oxygen production. J Membr Sci 276:178–184
Zenga P, Rana R, Chen Z, Gua H, Shao Z, Diniz JC, Liu Sh (2007) Significant effects of sintering temperature on the performance of La0.6Sr0.4Co0.2Fe0.8O3−δ oxygen selective membranes. J Membr Sci 302:171–179
Yang D, Lua H, Songa H (2004) Diffusion and permeation through YBa2Cu3O7−x membranes. J Membr Sci 233:45–50
Zhang K, Ran R, Ge L, Shao Z, Jin W, Xu N (2008) Systematic investigation on new SrCo1−yNbyO3−δ ceramic membranes with high oxygen semi-permeability. J Membr Sci 323:436–443
Bouwmeester HJM, Burggraaf AJ (1997) Dense ceramic membranes for oxygen separation. In: Gellings PJ, Bouwmeester HJM (eds) The CRC handbook of solid state electrochemistry. CRC, Boca Raton, USA, pp 481–553
Mitchell BJ, Rogan RC, Richardson JW Jr, Ma B, Balachandran U (2002) Stability of the cubic perovskite SrFe0.8Co0.2O3−δ. Solid State Ion 146:313–321
Tong J, Yang W, Zhu B, Cai R (2002) Investigation of ideal zirconium-doped perovskite-type ceramic membrane materials for oxygen separation. J Membr Sci 203:175–183
Liu J, Co AC, Paulson S, Birss VI (2006) Oxygen reduction at sol–gel derived La0.8Sr0.2Co0.8Fe0.2O3 cathodes. Solid State Ion 177:377–387
Mukasyan A, Costello C, Sherlock KP, Lafarga D, Varma A (2001) Perovskite membranes by aqueous combustion synthesis: synthesis and properties. Sep Purif Technol 25:117–126
Taheri Z, Nazari K, Safekordi AA, Seyed-Matin N, Ahmadi R, Esmaeili N, Tofigh A (2008) Oxygen permeation and oxidative coupling of methane in membrane reactor: a new facile synthesis method for selective perovskite catalyst. J Mol Catal A: Chem 286:79–86
Kameli M, Nazari K, Zare M (2007) New EDTA derivatives, their synthesis and application. European Patent Office, EP 1 808 428 A1
Yashima M, Kamioka T (2008) Neutron diffraction study of the perovskite-type lanthanum cobaltite La0.6Sr0.4Co0.8Fe0.2O3−δ at 1260°C and 394°C. Solid State Ion 178:1939–1943
Mcintosh S, Vente JF, Haije WG, Blank DHA, Bouwmeester HJM (2006) Phase stability and oxygen non-stoichiometry of SrCo0.8Fe0.2O3−δ measured by in situ neutron diffraction. Solid State Ion 177:833–842
Cullity BD (1978) Elements of X-ray diffraction, 2nd edn. Addison-Wesley Publishing Company, Inc, London
Ge L, Zhou W, Ran R, Liu Sh, Shao Z, Jin W, Xu N (2007) Properties and performance of A-site deficient (Ba0.5Sr0.5)1−xCo0.8Fe0.2O3−δ for oxygen permeating membrane. J Membr Sci 306:318–328
Shao Z, Yang W, Cong Y, Dong H, Tong J, Xiong G (2000) Investigation of the permeation behavior and stability of a Ba0.5Sr0.5Co0.8Fe0.2O3−δ oxygen membrane. J Membr Sci 172:177–188
Acknowledgment
The financial support of the Research Institute of Petroleum Industry is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taheri, Z., Nazari, K., Seyed-Matin, N. et al. Comparison of oxygen permeation through some perovskite membranes synthesized with EDTNAD. Reac Kinet Mech Cat 100, 459–469 (2010). https://doi.org/10.1007/s11144-010-0158-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-010-0158-2